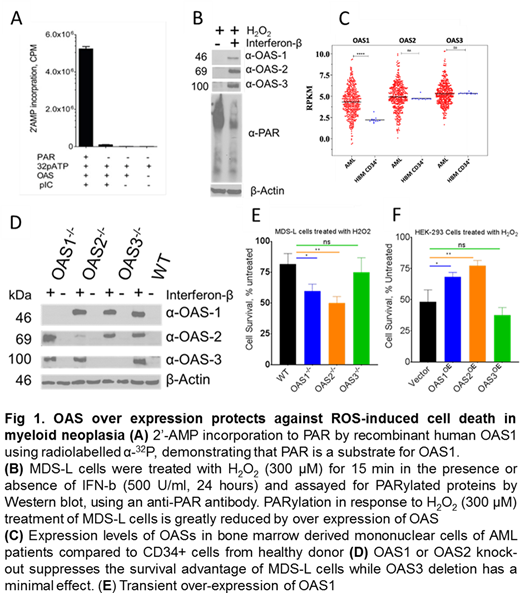
Acute myeloid leukemias (AML), the most lethal forms of blood cancer, are genomic instability disorders primarily driven by somatic mutations that greatly impact proliferation and survival of mutant clones1. The multistep mechanism of disease progression can be attributed, in part, to the defects in one or more pathways involving responses to, or repair of, damaged DNA (DD) and base excision repair (BER). The basal levels of DD and BER are higher in myeloid leukemias due to higher levels of reactive oxygen species (ROS)-mediated accumulation of 8-oxyguanine (8-OG)2. Poly(ADP) Ribosylation (PARylation) by PAR polymerases (PARPs) is one of the early key steps in sensing and repairing ROS-induced DNA damage response (DDR) 3. Consistent with recent findings4,5 here we report that 2'-5'oligoadenylate synthases (OASs), a family of latent 2'-5'-adenylyl transferases, otherwise involved in cellular antiviral responses6, are also involved in PAR remodeling of the DDR in MDS and AML cells. The 2'-hydroxyl group of PAR ribose acts as an acceptor for 2'AMP in the OAS enzymatic reaction and terminates chain elongation (Fig. A). OAS1 over expression can increase genomic instability by increasing the flux of PARP-mediated DNA repair, promoting cellular proliferation (Fig. B)5.
Expression analysis of OASs in AML patients (n=451, Beat AML) showed that OAS1 is upregulated (2-fold) in AML patients compared to normal bone marrow-derived CD34+ hematopoietic stem and progenitor cells (HSPC)(Fig. C). Knockout of OAS1 and OAS2 using Crispr-Cas9 in MDS-L cells (Fig. D), a cell line derived from an MDS patient, confers sensitivity to H2O2-induced DNA damage-mediated cell death; however, OAS3 has no effect. Conversely, ectopic over expression of OAS1 or OAS2 but not OAS3 in HEK293 cells provides protection against H2O2-induced cell death (Fig. E-F). Proteomic analysis of the OAS1 and OAS2 interactome using LCMS/MS suggests that over expression of OAS1 and OAS2 perturbs the differentiation program of HSPCs that may result in neoplastic evolution. Thus, OASs modify PAR chains, promoting speedy DNA repair and cell survival along with the induction of a differentiation block in HSPCs that leads to clonal expansion.
In summary, an overburdened DDR may contribute to AML pathogenesis. Therefore, inhibiting this stimulator of BER/DDR can provide a novel therapeutic avenue in myeloid neoplasms. The current study points to the probable utility of a novel therapeutic approach of targeting OAS in combination with DNA damaging agents to prevent relapse and resistance in the treatment of leukemias.
References
1. Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559(7714):400-404. doi:10.1038/s41586-018-0317-6
2. Jankowska AM, Gondek LP, Szpurka H, Nearman ZP, Tiu RV, Maciejewski JP. Base excision repair dysfunction in a subgroup of patients with myelodysplastic syndrome. Leukemia. 2008;22(3):551-558. doi:10.1038/sj.leu.2405055
3. Rogge RA, Gibson BA, Kraus WL. Identifying Genomic Sites of ADP-Ribosylation Mediated by Specific Nuclear PARP Enzymes Using Click-ChIP. Methods Mol Biol. 2018;1813:371-387. doi:10.1007/978-1-4939-8588-3_25
4. Khodarev NN, Minn AJ, Efimova EV, et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 2007;67(19):9214-9220. doi:10.1158/0008-5472.CAN-07-1019
5. Kondratova AA, Cheon H, Dong B, et al. Suppressing PARylation by 2',5'-oligoadenylate synthetase 1 inhibits DNA damage-induced cell death. EMBO J. 2020;39(11):e101573. doi:10.15252/embj.2019101573
6. Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res. 2011;31(1):49-57. doi:10.1089/jir.2010.0120
Saunthararajah:EpiDestiny: Consultancy, Current equity holder in private company, Patents & Royalties: University of Illinois at Chicago.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal